Lupin Pharmaceuticals has received the nod from the US FDA for its Minocycline hydrochloride extended release tablets 45 mg, 55 mg, 90 mg and 135 mg strengths.
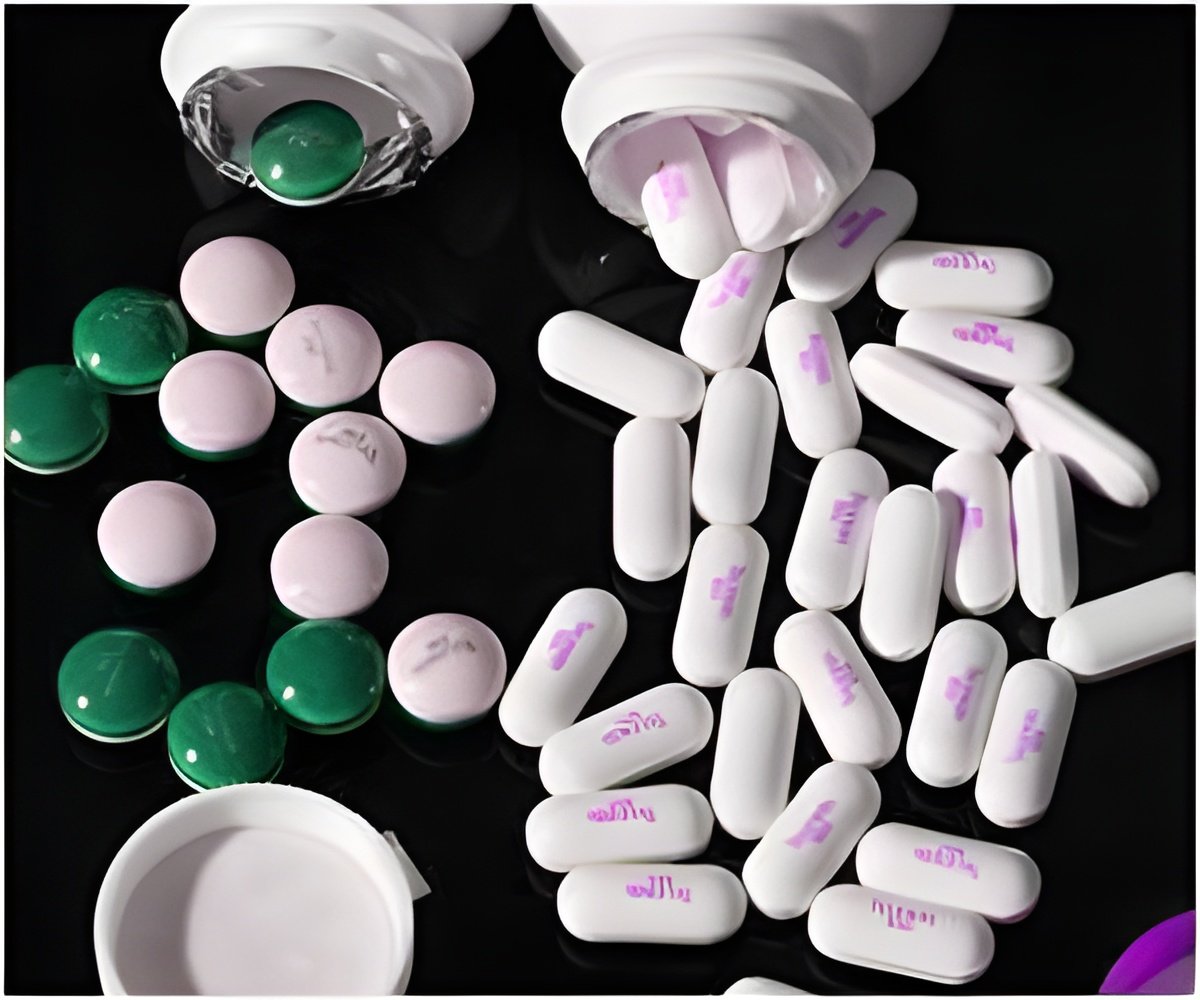
Solodyn is an oral antibiotic used in the treatment of red, pus-filled pimples and acne in patients 12 years of age and older.
Source-Medindia