The new digital radiography system is a ceiling digital X-ray system that offers streamlined workflows, diagnostic confidence and improved total cost of ownership.
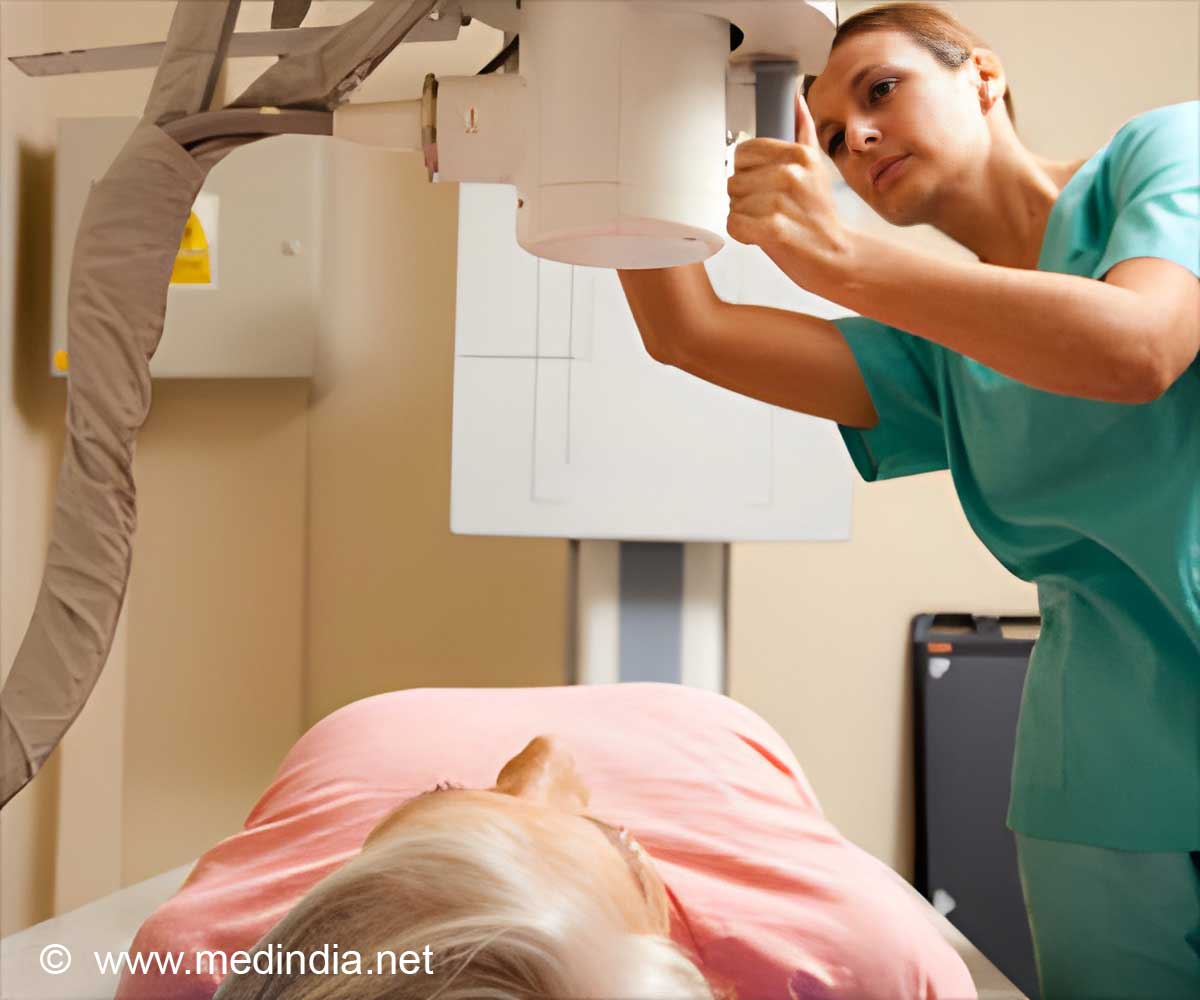
According to the company, the DR systems of the company combine advanced imaging technology, and user-friendly automated workflows to meet the demands of 21st century radiology departments.
The Samsung GC85A is a ceiling digital X-ray system that offers streamlined workflows, diagnostic confidence and improved total cost of ownership.
The new system’s S-Vue imaging engine provides dependable and accurate diagnoses in various conditions with reliably clear images, enhanced sharpness and clarity.
Another feature is wireless, lightweight s-detector with high detective quantum efficiency and a portable grid that displays the patient’s body structure clearly without increasing dose.
Other features include smart control to precisely position the entire system with one touch of the control panel’s buttons, s-align for precise alignment for exceptional imaging and smart stitching that eliminates the need for the patient to stand for long periods of consecutive shooting.
Advertisement
Advertisement