The drug, Nucala is administered once every four weeks by subcutaneous injection into the upper arm, thigh, or abdomen.
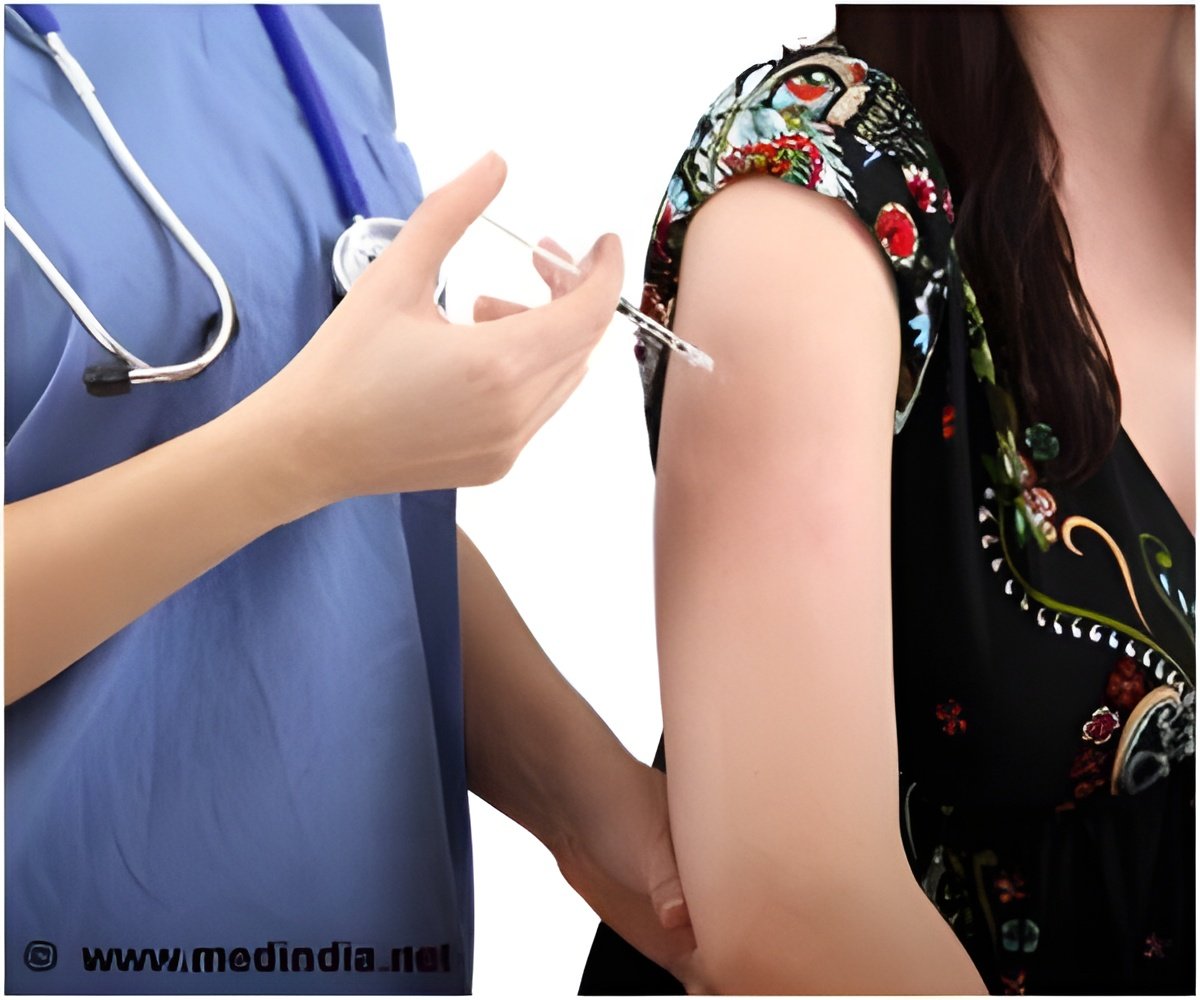
‘Nucala, an injected drug to treat severe asthma attacks has been approved by the US Food and Drug Administration. However, this drug has side effects like headache, injection site reactions, back pain, and fatigue.’





Nucala will be administered once every four weeks by subcutaneous injection into the upper arm, thigh, or abdomen.Nucala is a genetically engineered antibody is grown in cells from Chinese hamsters. The drug reduces severe asthma attacks by reducing the levels of white blood cells that contribute to the development of asthma.
Patients are advised to take other medications, including high-dose inhaled corticosteroids and at least one additional asthma drug.
The side effects of the drug include headache, injection site reactions, back pain, and fatigue. Hypersensitivity reactions can occur within hours or days of injection with Nucala.
Source-Medindia