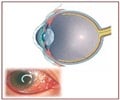
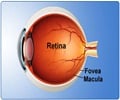
After getting the nod from the FDA for CR-2 PLUS digital non-mydriatic retinal camera, Canon has announced its release in the U.S. market.
The CR-2 PLUS employs a common Rebel digital SLR which is used as the image sensor.
The CR-2 PLUS camera enables capture of images, processing and storing them in a permanent storage base. This is possible due to Canon’s Retinal Imaging Control Software (RICS). Images can also be printed.
Source-Medindia