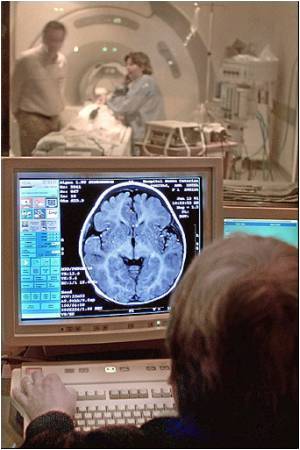
Brain death disrupted the levels of important molecules in the body called S-nitrosothiols that control many functions like oxygen delivery and cell activity.

‘Treating brain dead organ donors with S-nitrosothiols (SNO) medication may help improve tissue function in subsequent transplant recipients.’





Reynolds and colleagues earlier found that brain death disrupted the levels of important molecules in the body called S-nitrosothiols, or SNOs. SNOs control many crucial functions including oxygen delivery, blood flow, and cell activity. SNO deficiency can result in tissue injury and oxygen deprivation in the donor, reducing the likelihood of successful function of the transplant in the recipient. "Transplantation saves lives," said Reynolds, "but a successful outcome is highly dependent upon the physiologic status of the donor. Brain death can disturb SNO functioning, which we believe is a major contributor to procurement rates of suitable organs as low as twenty percent from consented donors. This is a disheartening figure when one considers how much demand outstrips availability. Clearly, better methods are needed to support the donor after brain death, and we will be exploring one such option under this grant."
Using a drug that grew out of the pioneering work of Reynolds' colleague Jonathan Stamler, (director of the Case Institute for Transformative Molecular Medicine), the Reynolds team had previously found that SNO deficiency in animals could be reversed, improving oxygen delivery and reducing tissue injury. With the new funding, they will use additional animal models to examine if treating donors with their proprietary compound improves tissue function in subsequent transplant recipients.
Specifically, they will be focusing on vascular composite allotransplantation, or VCA, the removal of a block of tissue from a donor and its attachment to a recipient. The tissue block is made up of skin, muscle, and bone, hence use of the term "composite." VCA is becoming an accepted method for correcting composite tissue loss (e.g. limb amputation) or catastrophic injury such as abdominal trauma and severe facial damage.
There are about 1,700 US amputee service members and 600 with severe facial disfigurement as a result of the Afghanistan and Iraq conflicts. "While some individuals will opt for prosthesis and grafts of their own tissue, our goal is to present VCA as a safe, viable option for replacing limbs and aiding in correcting devastating facial and trunk damage," said Reynolds.
Advertisement
"If our supposition holds true, we will find that muscle and tissue in human donors, not just animals, are also harmed by deficiencies in SNOs," said Reynolds. "If this is confirmed, in combination with positive results from our drug study in animals, support will be in place for a follow-on clinical trial with human VCA transplants. Then we could enact trials of the medication in standard organ transplantation as well. But for this to truly work, we need more organ donors along with these methods to increase the number of viable organs that can be obtained from currently consented donors."
Advertisement
Source-Newswise