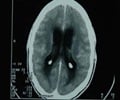
According to the Morbidity and Mortality Weekly Report, April 6, 2006, three more cases of Guillain-Barre syndrome (GBS), have been reported in people given the Menactra vaccine to prevent meningitis. This adds up to the five previous ones.
Still, when the eight cases of Menactra-related GBS were compared against the expected rates of GBS in populations in the same age group, no significantly increased risk was seen, suggesting that the association may have been due to chance alone.The possible link between the vaccine and GBS first surfaced in October 2005.
The first case involved a 19-year-old man who began experiencing numbness and weakness in his extremities, difficulty running, and decreased dexterity 25 days after being vaccinated with Menactra. Test results were consistent with GBS, and other possible causes of the neurologic symptoms were ruled out. He was treated and had made a full recovery by eight weeks after symptoms began.
The second case, which involved a 17-year-old male, was similar to the first, but disease onset occurred just 11 days after he was given the Menactra vaccine. Treatment resulted in a complete recovery two weeks after he has admitted to the hospital.