Esophageal tumor treated with genetically modified virus can be rendered sensitive to radiation, allowing for the synergy of these two therapies.
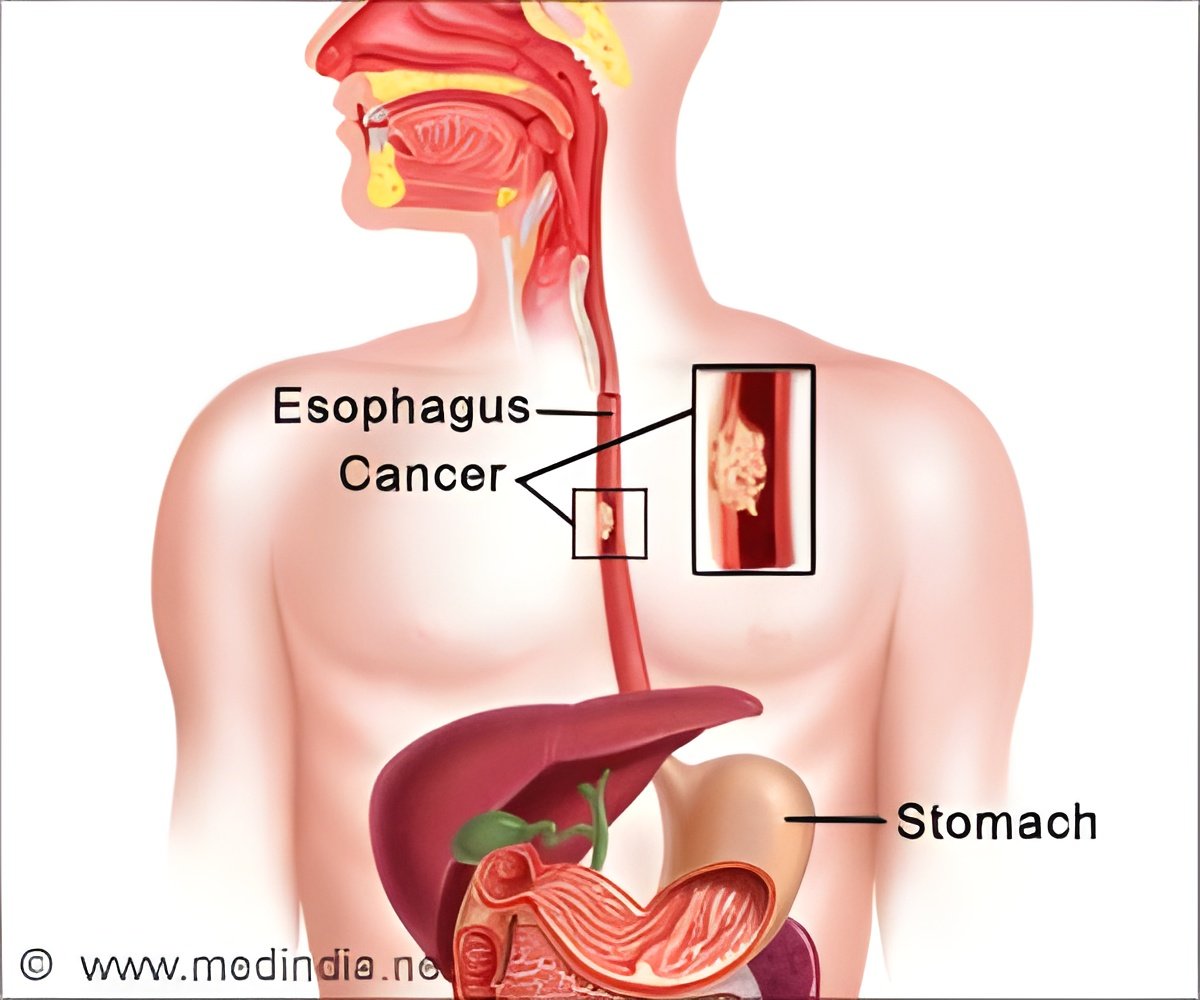
‘Combination of Adenovirus injection with radiotherapy is safe and induce complete eradication of esophageal tumors especially among patients who are not fit for receiving chemotherapy or standard therapy.’
Read More..




Many cancer cells overexpress the enzyme telomerase, which enables their replication and proliferation, explained Fujiwara. OBP-301 is a genetically modified virus which selectively replicates in telomerase-positive cells; viral infection is therefore enriched in cancerous cells, causing cell lysis, he added. A prior phase I trial evaluating OBP-301 as a monotherapy found the strategy safe and biologically active in patients with advanced solid tumors. Read More..
Because OBP-301 can also disrupt pathways that control DNA repair, Fujiwara and colleagues believe that tumor cells treated with the virotherapy can be rendered sensitive to ionizing radiation, allowing for the synergy of these two therapies.
In this open-label, phase I dose-escalation trial, the investigators enrolled 13 patients with histologically confirmed esophageal cancer deemed unfit to receive standard surgery or chemotherapy; all patients were elderly and/or those who had complications such as pulmonary disease, cardiovascular disorders, or liver dysfunction, explained Fujiwara. Patients' median age was 80 years.
Patients received three intratumoral injections of OBP-301 (each injection ranging between 10 billion and 1 trillion viral particles) via endoscope over several weeks with concurrent radiation therapy to a total of 60 Gray. The primary and secondary endpoints were dose-limiting toxicities and objective response rate (ORR), respectively.
In all 13 patients, the ORR was 85 percent, which included eight complete responses (CR) and three partial responses (PR); the clinical CR rate, defined as endoscopically and pathologically confirmed complete disappearance of tumor in the esophagus, was 83 percent and 60 percent for patients with stage 1 and stage 2/3 disease, respectively. No patients had viable malignant cells in post-treatment biopsy specimens.
Advertisement
"Not only was our combinatorial therapy safe, but it induced the complete eradication of esophageal tumors in most patients," said Fujiwara. "These preliminary results indicate that there may be less invasive treatment options available for esophageal cancer patients who are unable to receive standard therapies for their disease."
Advertisement
Limitations of this study include small sample size, which is consistent with a phase I dose-escalation trial.
Source-Eurekalert