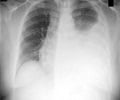
India is left with 32 months (2025) to end tuberculosis and Nigeria is left with 92 months (2030) respectively.

Century-old TB Vaccine and Still not 100% coverage in Hard-hit Nations
The only vaccine against TB, the 102 years old BCG (Bacille Calmette Guerin), has a protective effect against meningitis and disseminated TB in children. It does not prevent primary infection and does not prevent reactivation of latent pulmonary infection. It is hard to fathom that why for a disease that was the deadliest infectious disease in the world before COVID-19 hit us, why better vaccines to prevent and control TB are not around yet? More importantly, despite 102 years of BCG vaccine being around, its coverage is still not 100% in TB hard-hit nations like India and Nigeria - as per WHO data, BCG vaccine coverage in 2021 was 84% in India and 75% in Nigeria. Science first: “While the neonatal BCG vaccination is partially effective in reducing the risk and severity of serious forms of TB, like miliary TB and TB meningitis in infants and young children, it is poorly protective against pulmonary disease in adolescents and adults. There is a critical need for new TB vaccines that are more effective than the existing BCG vaccine in preventing pulmonary as well as extra-pulmonary forms of TB in all age groups,” said Prof Surya Kant, scientific Chairperson of 77th National Conference of Tuberculosis and Chest Diseases held in Agra, India.‘A new tuberculosis vaccine currently in Phase-3 study in India if proven safe in human trials will be released to the market soon.’





BCG vaccine is contraindicated in those with cell-mediated immune deficiency. While HIV infected, non-symptomatic infants should be immunized with BCG vaccine according to standard schedules, infants with clinical (symptomatic) AIDS should not receive it.
First BCG Shot in 1921: Why Did it Take Decades to Scale up its Rollout?
BCG vaccine was first administered in humans in 1921. Despite the crippling burden of TB, it took almost another half a century for governments to begin resolving issues, which marred its rollout in TB affected nations, including manufacturing and supply-chain related issues.“In 1970s and early 1980s, supply of BCG vaccine was very erratic for a very long time. So, we did not have much vaccine stock. BCG immunization picked up only after mid-1980s,” said Dr. Jacob John, Professor, Community Health department, Christian Medical College (CMC) Vellore, India. Vaccine shortage was so acute back then that “we had to import it from Denmark. Moreover, as it is not easy to maintain these doses, there was lot of wastage of the doses,” said Dr. John.
“In those days not just BCG vaccine uptake was low, but all other vaccination rates were low too. Institutional delivery rate was also low. Institutional delivery rates began picking up in mid-1980s,” said Dr. John. “BCG vaccination is now a part of institutional deliveries – when babies are born people know they have to give them vaccines, including BCG vaccine.”
Agrees Dr. Shibu Vijayan, a noted public health crusader who currently serves as Medical Director (Global Health) at Qure.ai. “Births that earlier happened at homes are now happening at medical facilities. The route of BCG vaccine is a health facility-based function. So, if the baby is not delivered in a medical facility, he/ she/ they may not get the vaccine.”
A similar situation thrives in Nigeria: The nurse in charge of primary health centre (PHC) Angwan Maikai, Keffi, Nasarawa State in Nigeria, said that, like other PHCs, mothers are informed of the various vaccines, including BCG, during antenatal care. The challenge comes for those who do not attend antenatal or delivery at health facilities.
Advertisement
A TB case worker with APIN Public Health Initiative, Mercy Depuun noted that the uptake of vaccines in Nigeria has been generally low because of hesitancy from the public, and this was because of a lack of trust in the vaccines. There were various myths surrounding vaccines in this part of the world, including getting infected with diseases from taking the vaccine, life-threatening side effects from the vaccine, and birth control plots by the west.
Advertisement
Mercy further observed that generally this would not be an issue as the government agency responsible for the expanded programme on immunisation has some mechanism to deal with it. A massive vaccination campaign has addressed this for all childhood vaccinations, including BCG. There was some house-to-house vaccination of children eligible for BCG vaccine. Also, the eventual lifting of movement restrictions imposed due to COVID-19 has increased and improved access to health facilities for parents of infants.
Among the people who administer BCG vaccines in India are frontline community health workers or ASHA (Accredited Social Health Activist) workers. We spoke with Reshma Adagale, who is an ASHA worker in Maharashtra state of India and a part of Nagpur Municipal Corporation Employees Union: “During the 6-7 years of my working as ASHA worker, I have not had to convince much, as most people know it is important for children to get BCG vaccination and they are willing to get their babies vaccinated. Very few (around 1-2%) need to be counselled about its benefits or any hesitation they may have.
They may need to be informed about its public health benefits, about possible side effects, etc. Some people make flimsy excuses for not getting their child vaccinated, like ‘when children get injection they keep crying for a long time; or kids get fever after vaccination and then parents have to leave their work and take the kid to the hospital (which results in loss of wages)’; or that ‘we will get our child vaccinated if the family elders permit us to do so’; or ‘we did not get this vaccine in the past , so why should our child get it’. These are some myths and misconceptions we come across at times. We try our best to convince them and get their kids vaccinated.” However, she confirmed that she had to work hard with people to counter vaccine hesitancy against COVID-19 vaccine.
Bhagyashree, another ASHA worker in Nagpur, India, told us that in the past 5 years of her work life, she has not encountered any vaccine hesitancy against BCG. Like Reshma, she said that COVID-19 vaccine hesitancy was there. “I had to convince them on vaccine benefits, educate them on side effects, and bust myths and misconceptions like ‘it will make us impotent’ etc, and eventually we got our whole area vaccinated against COVID-19.”
“We need to recognize the role of Auxiliary Nurse Midwives (ANMs) and ASHA workers who have been going door-to-door around the villages in raising awareness and health education about importance of these vaccines and other health interventions since years. Ultimately it is about involvement of healthcare workers and health literacy in the field. People may not know what is ‘BCG,’ but they are likely to understand that its shot will help protect their child from disease in future. Role of healthcare workers on the ground is phenomenal,” said Jibin TC, National Working Secretary of United Nurses Association.
In May 1948, Indian Government had issued a press note stating that TB was “assuming epidemic proportions,” and that it had “after careful consideration” decided to introduce BCG vaccination. Government-run BCG Vaccine Laboratory was set up in Chennai, India, in 1948, which continues to produce this vaccine till to date.
BCG vaccine was introduced and has been used in Nigeria since 1921. However, in India the first BCG shots were given in August 1948. Later when India’s National TB Control Programme (now called the National TB Elimination Programme) began in 1962, BCG vaccination became its part.
Since there were questions on efficacy of BCG vaccine in preventing TB of the lungs from the early days, a large BCG clinical trial (Feasibility Study for TB Prevention Trial) was conducted in Chingelput, Tamil Nadu, India, during 1968-1987. This study showed that BCG vaccination did not offer significant protection against TB of the lungs which occurs mostly in adults.
That is why BCG vaccination policy was revised in India, recommending BCG vaccine to be given to young children within first year of the birth, by integrating it under Universal Immunization Programme. Other countries tweaked their BCG vaccine policy too due to the study’s findings. BCG is now given to infants soon after birth or later, but preferably before exposure to persons with active TB disease. The same is the practice in Nigeria, where the BCG vaccine is given within the first week of birth.
In 1958, World Health Assembly of the WHO, had passed a resolution to eradicate smallpox, following which India started its National Smallpox Eradication Programme in 1962 with the goal to give smallpox jab to all population. But the uptake of this vaccine (which was discovered in 1778) remained low in the following years for myriad reasons.
India eventually became smallpox free in 1977 and Nigeria in 1980. It is important to note that in India the first smallpox vaccine shot was administered in 1802. But it took almost 200 years to make India smallpox free in 1977.
After achieving its ‘smallpox-free’ milestone, India launched a nationwide Expanded Programme of Immunization in 1978 which included the goal to vaccinate 80% of infants in major urban hospitals with BCG vaccine. As was expected, coverage remained abysmally low. In 1985, India’s Expanded Programme of Immunization evolved as Universal Immunization Programme with major changes and a broader scope.
After about five decades of its setup, it was only in 2000-2001, when Indian government’s BCG Vaccine Laboratory was producing enough BCG vaccines to meet 100% requirement of Universal Immunization Programme, as well as to export the vaccine to other nations in need. Consequently, it stopped import of BCG vaccine. Private BCG manufacturers, including Serum Institute of India, began BCG vaccine production in 2002 onwards.
“Current BCG coverage is high in most parts of India. If there are better vaccines people will take it - if its safety and efficacy is not in doubt. That is because a general idea that ‘vaccines are good for infants’ is accepted. But when we talk about vaccines for adults and/or older children (more than one year of age), then there are a whole lot of reasons why people may not want to opt for it,” said Dr. Jacob John. “Reluctance in some people to take adult vaccination or get older children vaccinated is more of a sociological issue than hesitancy.”
We spoke to some leaders who are major influencers of TB response in India. “The existing BCG vaccine is a part of the universal immunization programme and is given to all infants born on day-zero. But the new vaccines that are being researched will hopefully be applicable to a larger population, if proven to be safe and effective. Research is currently underway so we will hopefully get to know soon if these new TB-vaccine candidates under research will be prioritised for most-at-risk populations, household contacts of TB patients, and for re-vaccination of those who got BCG shots earlier. The last word on the new vaccine(s) is not yet out to be used on a programmatic scale,” said Dr. Kuldeep Singh Sachdeva, former head of India’s national TB programme who has been deeply involved in the fight against TB for around two decades now. He currently heads the Southeast Asia regional office of International Union Against Tuberculosis and Lung Disease (The Union).
There are several new TB vaccine candidates that are currently under different stages of research. Dr. C Padmapriyadarsini, who is a noted scientist and the Director of government-run National Institute for Research in Tuberculosis. (NIRT), Indian Council of Medical Research, said: “NIRT is involved with the study of recombinant BCG vaccine, which is a TB preventive (prophylaxis) vaccine meant for the household contacts of TB patients. This study has been going on for almost 3 years now and is being done at 12 sites across India. In the study, household contacts of TB patients (who are above 14 years of age) were screened for TB and those who tested negative for TB disease, were given this TB preventive vaccine. There was a follow up period of 2 years to see whether they developed (or not developed) TB. While we are expecting good results, the interim results will be available by end of March 2023 or early April 2023. Only then will it be known if this vaccine is helpful in preventing TB.”
The recombinant BCG vaccine is also being tested in Bangladesh (and a few centres in India) to see if it can prevent TB recurrence when given to treated TB patients. Results of this are also expected around end of March 2023 or early April 2023.
India is also planning a study to investigate BCG revaccination or BCG vaccination in adult population. “We will investigate whether the BCG vaccine (that is currently used to vaccinate infants) can be used for adult vaccination to prevent TB. Some countries are already trying this BCG vaccine in adults to see whether it would prevent TB. A few small studies have shown that it increases the immunity of an individual,” said Dr. Padmapriyadarsini.
“There could be hesitancy when a new TB vaccine is introduced. And to overcome that and increase its acceptance we need to create awareness in the public. We saw a similar thing in COVID-19 vaccine where initially there was a lot of hesitancy. So, we must create awareness in the public that the vaccine is safe, and from what does it protect. That type of confidence building will go a long way in removing vaccine hesitancy and increase uptake of any vaccine,” rightly said Dr. Padmapriyadarsini.
Currently, feasibility and acceptability studies are also going on regarding the possible new TB vaccine in India, to find out if and why people might be hesitant to take it. “By the time the vaccine is ready we will have these results also. It would help if the vaccine became a part of the government vaccine immunization programme. For faster uptake, vaccine rollout must be a joint venture involving the manufacturer, government, public and private sector, and all other stakeholders who can help,” said Dr. Padmapriyadarsini.
Dr. Sachdeva agrees: “We will have to take along other stakeholders to help improve the acceptance of a possible new TB vaccine (currently under research) and scale up its uptake in the targeted populations. An important element for the roll out of new TB vaccine will be capacity building of the healthcare providers and the community. Lessons learned from COVID-19 vaccination will go a long way to reduce vaccine hesitancy to a large extent in the rollout of any new vaccine for TB or any other disease in the future.”
Not only was the TB programme adversely impacted due to the COVID-19 pandemic, but there was also a decline of TB funding from US$ 5.8 billion to US$ 5.3 billion, which resulted in a drop in the number of children who got BCG shots.
If we are to end TB, we must stop neglecting TB prevention – be it infection control or other measures that reduce risk of getting active TB disease. A safe and effective TB vaccine for all age groups is an important cog-in-the-wheel of the TB combination prevention cascade.
Source-CNS