- History of evidence-based medicine - (https://www.ncbi.nlm.nih.gov/pmc/articles/pmc3263217/)
- Evidence-Based Practice: 5 steps of Evidence Based Practice - Overview - (http://library.health.nt.gov.au/ebp/overview)
- Meta-analysis in medical research - (https://www.ncbi.nlm.nih.gov/pmc/articles/pmc3049418/)
- About The Cochrane Collaboration - (http://consumers.cochrane.org/about-cochrane-collaboration)
- Evidence Based Medicine – New Approaches and Challenges - (https://www.ncbi.nlm.nih.gov/pmc/articles/pmc3789163/)
- Evidence-based medicine: Classifying the evidence from clinical trials – the need to consider other dimensions - (https://www.ncbi.nlm.nih.gov/pmc/articles/pmc1751050/)
- Evidence-Based Practice In 5 Simple Steps - (http://www.jmptonline.org/article/s0161-4754(08)00096-1/pdf)
- Evidence Based Health Care - (https://www.ncbi.nlm.nih.gov/pmc/articles/pmc3074860/)
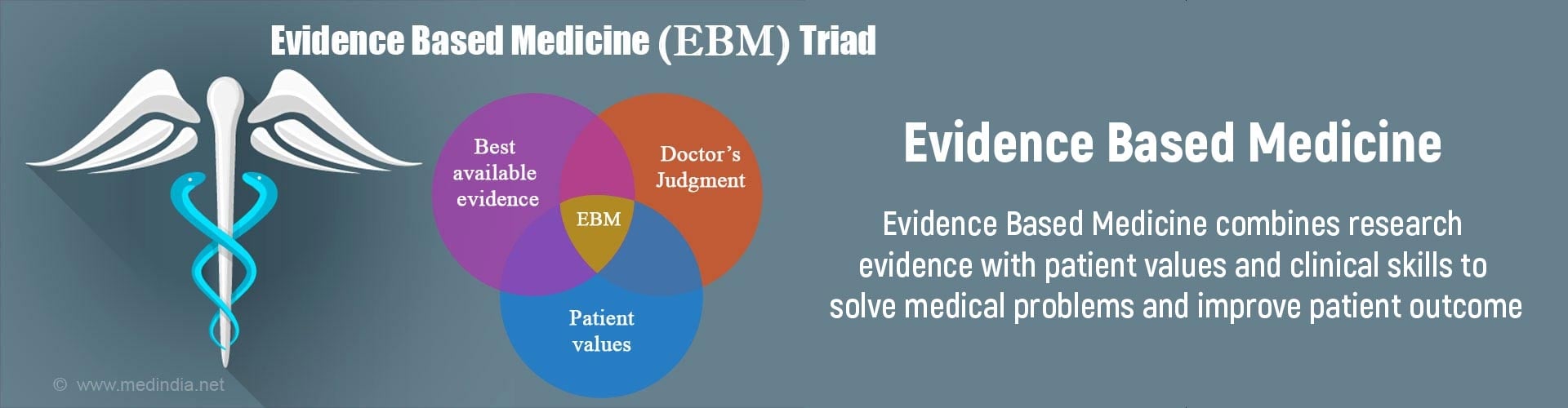
What is Evidence Based Medicine?
“EBM is nothing more than a process of life-long, self-directed learning in which caring for patients creates the need for clinically important information about diagnosis, prognosis, therapy, and other clinical and health care issues.” -- The EBM Working Group
Evidence based medicine is an integration of individual clinical expertise, patient’s values and relevant clinical research to make the best decision for patient care. It is a systemic approach to optimize healthcare decision-making based on well-conducted clinical or research studies. These studies have properly randomised controls to show difference in the treatment outcomes.
The practice of evidence-based medicine is a process of self-directed problem-based learning in which clinically relevant information about diagnosis, prognosis, and therapy is considered according to the best data available through research.
History of Evidence Based Medicine
The term “Evidence-Based Medicine” (EBM) was coined in the year 1991 by Dr. Gordon Guyatt, a coordinator at McMasters University Internal Medicine residency. But, David Lawrence Sackett a clinical epidemiologist, and founding Chair of the Cochrane Collaboration is widely regarded as a pioneer and the father of evidence-based medicine. Sackett and his colleagues focused on the need to not only teach methods to understand the scientific literature but also to teach the application of new information to the physicians. It has been described as one of the most important medical advancements in the past 150 years, alongside the discovery of vaccines and antibiotics.
What is the Cochrane Collaboration?
The Cochrane Collaboration is a non-profit organization based in the UK. It comprises over 10,000 persons worldwide most of whom do not receive any payment for their work in supporting the organization’s activities.
The main mission of this organization is to go through and prepare systematic reviews of new research that is being published every day in various specialties based on the strongest evidence available, in order to help make informed decisions on healthcare interventions for the best possible patient outcome.
These reviews are then published electronically within The Cochrane Library and are freely accessible to healthcare professionals as well as consumers in abridged versions.
The Cochrane Library consists of several databases containing high-quality, independent evidence to help in making informed decisions about clinical interventions. Cochrane Reviews can be considered the topmost level of evidence based on which important treatment decisions might be made.
Evidence Based Practice in Healthcare
In order to make the best decisions for day to day patient care, valid information about prevention, diagnosis, prognosis and treatment is required. Implementing the Evidence-Based Practice (EBP) competencies in the healthcare setting improves healthcare quality and patient outcomes. Unnecessary treatment is avoided reducing costs. The need and demand for evidence based practice in healthcare is growing rapidly because of the following factors:
- Information overload i.e too much of information available
- Rising patient expectations
- New and advanced technologies
- Aging populations
EBM in healthcare is an approach to optimize decision making where a clinician uses the best evidence research in consultation with the patient to decide which option provides the patient the maximum benefit. The management of health care system makes policies in order to allocate funds, purchase or manage resources.
Classification and Levels of Clinical Evidence
EBM covers a broad range of topics including clinical epidemiology, biomedical informatics and guidelines to perform it. Therefore,the levels of clinical evidence are an important aspect of EBM. It helps the reader to prioritize the available information.
The levels of evidence were originally classified by the Canadian Task Force on the Periodic Health Examination in 1979. Subsequently, the levels were further elaborated by Sackettin an article about the levels of evidence for antithrombotic agents in 1989. Interestingly, both systems placed randomized controlled trials (RCT) at the top most level and expert opinions (case reports) at the bottom.
Levels of Evidence from Sackett
| I | Large Randomised Control Trials with clear cut results |
| II | Small Randomised Control Trials with unclear results |
| III | Cohort and case-control studies |
| IV | Historical cohort or case-control studies |
| V | Case series, studies with no controls |
*Adapted from Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 1989;95:2S–4S
How is Level of Evidence Graded?
The grading system is an important part of evidence-based medicine and helps in clinical decision making based on the quality of available evidence. For example, a strong recommendation is given when there is level I evidence and consistent evidence is available from Level II, III and IV studies. Thus the grading system does not ignore or neglect lower level evidence when decisions on recommendations are being made, provided the results are consistent.
Level 1 studies are well constructed studies and are “free from any bias that might otherwise be introduced by the people involved.” These are considered to be the gold standard studies for clinical practice.
Various authors and organizations have used different classification systems to grade the levels of evidence and strength of recommendations. Differences and flaws in the various grading systems have resulted in confusion and miscommunication.
To be useful and practical, grading systems need to be simple, clear and systematic where the quality of evidence and the strength of recommendations are concerned.
The GRADE system defines the 'quality of evidence' that the clinician is using to make a judgment. Here is the overview of grading system given to the clinical evidences.
GRADE System of Quality of Evidence and Explanation of the Categories
| Quality Rank | Evidence Source |
| High | Randomized trials without serious limitations along with well performed observational studies with very large effects |
| Moderate | Randomized trials with serious limitations but with well performed observational studies with large effects |
| Low | Randomized trials with very serious limitations and Observational studies without special strengths or serious limitations |
| Very low | Randomized trials with very serious limitations and inconsistent results. Observational studies with serious limitations. Unsystematic clinical observations eg case series or case reports |
What are the Factors Affecting Grade or Quality of Evidence?
In spite of the fact that RCTs (see table below) are often given the highest level of evidence quality, not all RCTs are performed as per the norms, and the results should therefore be carefully inspected. Sackett emphasized the importance of assessing the nature and types of errors and the strength of studies when interpreting results of RCTs.
GRADE Quality Assessment Criteria
| Type of Study | Quality of Evidence (Grade) |
| Randomized trial | High |
| Observational Study | Moderate to Very Low |
| Other Type of Evidence | Very low |
However, the grade may be lowered, for example, from high to moderate or high to low or conversely increased from moderate to high due to certain factors. Factors such as limitations in the study, inconsistencies in results and indirectness of evidence lower the quality of evidence. On the other hand occurrence of a large magnitude of effect or presence of a dose response gradient may enhance the quality of evidence and up the grade.
| Lower Grade If | Increase Grade If |
|
|
|
Grading evidence and recommendations. Health Res Policy Syst. 2006;4:21 (with permission).
Steps of Evidence Based Medicine
EBM is a systematic approach and an on-going process. Sackett et al originally described the basic principles and the 5 steps of evidence based practice (EBP). These principles followed are very similar to those followed in the continuous quality improvement (CQI) cycle.
Typically the CQI cycle starts with a specifically felt need and a desire for improving the existing quality. The next steps consist of searching for the most appropriate information to arrive at the possible solution, a careful assessment to make sure that the information/solution is correct, application of the most apt or suitable information/solution to the situation, followed by monitoring the effects of implementation practices to ensure the desired improvement or outcome has been achieved, and then lastly incorporating this solution into regular practice.
Consciously applying the above principle steps in clinical practice would help to improve patient care and outcome for the patient.
The five steps of EBM are as follows:
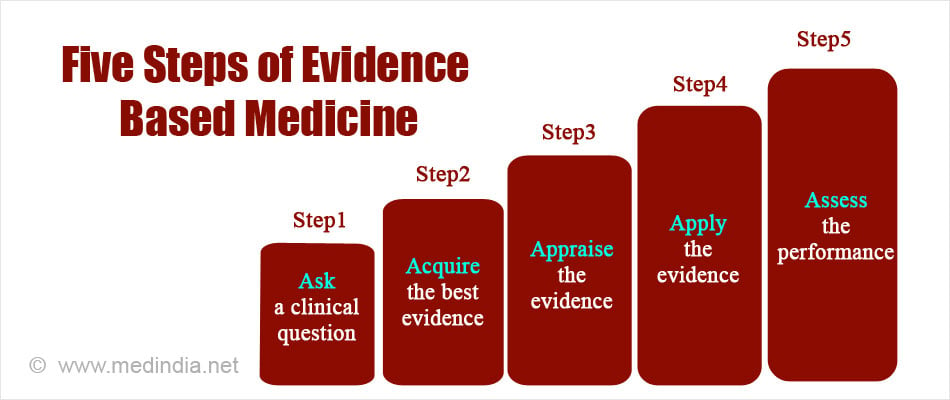
1. Asking an answerable question
The first step explains the need to improve the method of collecting information from a patient. A more detailed question should be formulated so that a clear answer is found in terms of prevention, diagnosis, prognosis and therapy.
2. Acquiring best evidence
High quality sources (Cochrane database etc.,) should be used for finding out the best possible answer to the question.
3. Appraising the evidence
This step involves critical appraisal and thoughtful evaluation of the evidence by the doctors. It helps to identify the validity and closeness of the information to the patient and/or clinical setting.
4. Applying the evidence
The fourth step is the application of the best clinically relevant information to the patient and/or clinical setting. The selected information is implemented and then observed for its effects.
5. Assessing the performance
The fifth step evaluates the outcomes of the information used for a particular case. It checks the effectiveness and efficiency of the information applied and possible improvements in the clinical practice.
Why it is Important to Use Evidence Based Practice?
- EBP is important because it aims to provide the most effective care that is available, with the aim of improving patient outcomes. Before EBP came into force, the health professionals relied on the advice of experienced colleagues, and often used their findings or intuition for taking decisions. However, this practice was not accurate as the information provided by older colleagues can be biased, incorrect, and not appropriate for a particular patient today. So, rather than relying on previous clinical experience alone for decision making, health professionals need to use clinical experience together with clinically relevant evidence-based information.
- EBP can be considered a preferred option over traditional medical training. EBP promotes an attitude of inquiry in healthcare professionals i.e. asking “is there any valid evidence that can be used in support to do this in a more effective way”?
- EBP also plays an important role in ensuring that health resources are used wisely and that only the best relevant evidence is considered when decisions are made about funding health policies. It is of utmost importance for physicians while handling critical and emergency situations.
What is Meta-analysis and Why is it Important for Level 1 Evidence?
Meta-analysis is the statistical procedure that combines available results and data from multiple studies. A common treatment effect (or effect size) that is consistent across many independent studies, can be identified by meta-analysis.
All important medical questions or problems are usually carefully assessed or studied more than once, by different research teams at different places in the hope of finding a solution. In many instances the results of these studies may be contradictory to each other, making clinical decision confusing and difficult. Therefore, meta-analysis which tries to integrate the results of many independent studies plays a key role in evidence based medicine.
In fact, in evidence based clinical practice, systematic review and meta-analysis of high quality well designed, randomized clinical trials free from bias is considered Level I evidence and placed right on top of the pyramid.

What are the Limitations of Evidence Based Practice?
- The Evidence Based Practice to get level 1 evidence requires large well-constructed randomized clinical trials and these are expensive to conduct and undertake.
- The RCT’s only represent a segment of population and what is true in one population group or segment ( depending on the race, and geographical location) may not apply in another group and may not be reproducible and representative.
- There can be a big lag time period between the RCTs and its application in the general population.
- There could be bias to the publication of RCTs by a journal. The industry could also have played a role in creating such bias.
Examples of Some Evidence Based Medicine that has Benefited Patients
The list below is by no means exhaustive but highlights how evidence from well conducted studies can offer the best possible outcome and avoids unnecessary interventions for patients.
- A 2010 meta-analysis of 13 studies concluded that only patients who test positive on a stool test need to undergo colonoscopy for further evaluation of suspected inflammatory bowel disease. Those who test negative can be reassured and avoid undergoing colonoscopy which is invasive and causes some pain and discomfort.
- A 2004 randomized trial showed that early steroid treatment offered best chance of recovery in Bell’s palsy.
- Steroids administered to mothers in premature labour save lives of premature babies, reduce the risk of respiratory distress syndrome by 50% and improve the babies’ longterm outcome.