Homeostasis and the Acid Disposal System of Our Body
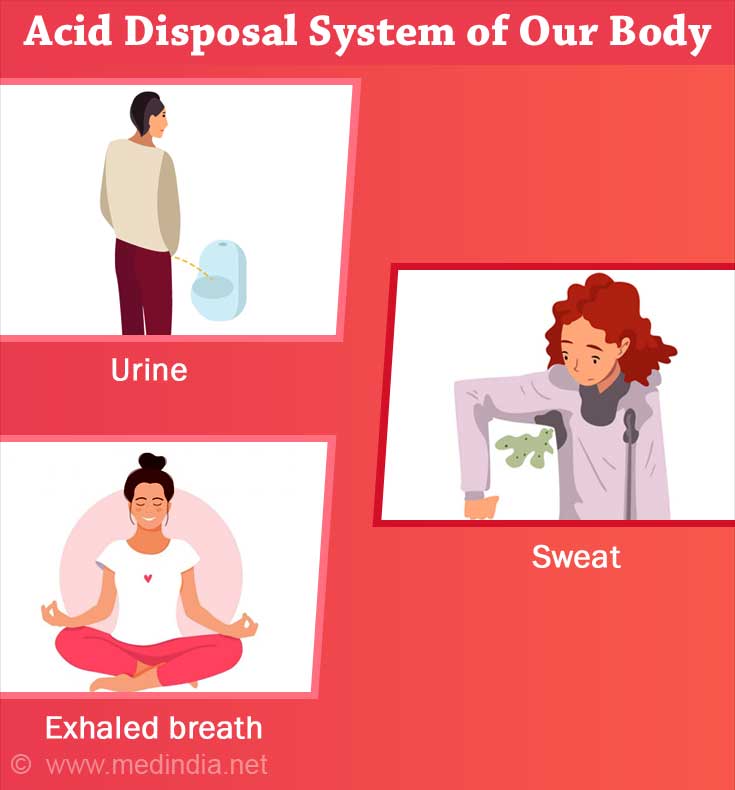
Body tries to get rid of the acids through urine, sweat and exhaled breath. LDL cholesterol, uric acid, fats add to the acid load and are bad for health.
In order to sustain human life, the body must maintain a constant body temperature and constant body fluid pH values. The process of maintaining pH values to within narrow range of 7.3 to 7.45 is complicated.
Our body tries to get rid of the acids through urine, sweat and exhaled breath. The kidneys excrete the excess acids in the urine (generally the pH of urine is acidic – from 5.5 to 6.5)., and the lungs eliminate acids in the form of carbon dioxide that we exhale.
A glass of cola with its pH value of 2.5 can change 10 gallons of water with a pH of 7.4 into water with a pH of 4.6. As we drink highly acidic drinks the blood becomes acidic instantaneously and the body must act quickly to restore the pH values back to the safe range.
Carbon dioxide is formed by the breaking up of carbonic acid in the body to make it less acidic. Carbonic acid is volatile; therefore it is exhaled to make the blood alkaline. Another way the body prevents increased acidity is through alkaline blood buffers such as sodium bicarbonate and alkaline sodium phosphate.
The kidneys and lungs have a certain capacity of handling the acid wastes. Bharti Vyas in her book "The pH balance diet" states that long standing acidic abuse to the body often exhausts it which then renders it unable to breakdown the wastes from acid forming foods, drinks and stimulants completely. When this occurs, substances that cannot be processed are stored somewhere in the body.
She also states that, in order to live healthily our blood and cells must always remain slightly alkaline. So the body always changes leftover acid wastes into harmful solid wastes (such as LDL cholesterol, uric acid, fats) and stores them.