- Bietti crystalline dystrophy - (http://ghr.nlm.nih.gov/condition/bietti-crystalline-dystrophy)
- Halford S et al. Detailed Phenotypic and Genotypic Characterization of Bietti Crystalline Dystrophy. Ophthalmology 2014; 121(6):1174-84. doi: 10.1016/j.ophtha.2013.11.042
- About Bietti Crystalline Dystrophy - (http://www.ncbi.nlm.nih.gov/books/NBK91457/)
- Mansour AM, Uwaydat SH, Chan CC. Long-term follow-up in Bietti crystalline dystrophy.Eur J Ophthalmol. 2007 Jul-Aug;17(4):680-2.
- Wilson DJ, Weleber RG, Klein ML, Welch RB, Green WR. Bietti''s crystalline dystrophy. A clinicopathologic correlative study. Arch Ophthalmol. 1989 Feb;107(2):213-21.
- Kojima H1, Otani A, Ogino K, Nakagawa S, Makiyama Y, Kurimoto M, Guo C, Yoshimura N. Outer retinal circular structures in patients with Bietti crystalline retinopathy. Br J Ophthalmol. 2012 Mar;96(3):390-3. doi: 10.1136/bjo.2010.199356. Epub 2011 Jul 29.
About
Bietti crystalline dystrophy (BCD) is a condition in which lipid deposits accumulate in the retinal tissues of the eye leading to progressive loss of vision.
BCD involves chorioretinal degeneration and is characterized by deposits of yellowish white crystals in the retinal pigment epithelium (RPE). The retina is the light-sensitive inner layer of the eye, while the choroid is the vascular and pigmented middle layer. Those affected begin to experience problems while in their late teens or early twenties when there is a reduction in vision acuity. Eventually, their field of vision becomes narrowed and there is a loss in peripheral vision. The progress of the symptoms varies with individuals, even between family members.
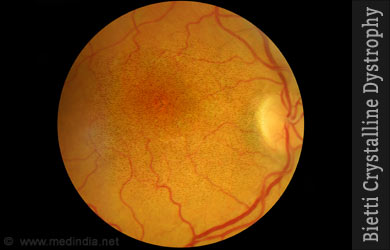
Mutations in a single gene CYP4V2 are known to cause BCD. This gene is responsible for producing one member of the cytochrome p450 family of enzymes which is involved in many tasks, including oxidation of lipids. Any mutation in CYP4V2 affects lipid break down which in turn could be responsible for the development of BCD. The exact dynamics are not clear, as those with the same mutations present with different sets of symptoms.
BCD is passed on through generations in an autosomal recessive manner. For a couple who are recessive for the condition, there is a 25% chance of producing an affected child, 50% chance of having an asymptomatic carrier, and a 25% chance of producing an unaffected child who is not even a carrier.
BCD is quite rare and is more common among those of East Asian descent, such as the Japanese and the Chinese. There are many variants of the condition and several new ones are now being reported. One variant - an early onset type - has been seen in some individuals of South Asian descent.
Clinical features
Some of the features of BCD include the following:
- Progressive atrophy and degeneration of RPE /choroid
- Sclerosis or thickening of the choroid vessels
- Pigment clumps
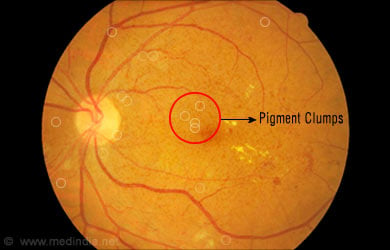
- Reduced clarity in vision
- Marked asymmetry between both eyes
- Poor night vision
- Visual field loss
- Variable crystalline deposits around the cornea
- Impaired color vision
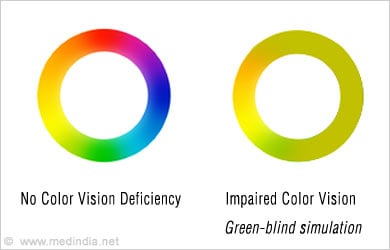
- Impaired retinal electrophysiology on testing
Diagnosis and Management
Diagnosis is established once numerous small, glistening yellow-white retinal crystals are detected, mostly through the optical coherence tomography (OCD) method. Other features that help in diagnosis include the following:
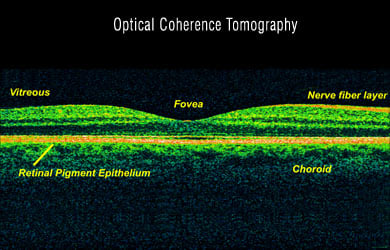
- Crystalline deposits in the corneal limbus (the part around the cornea)
- Electroretinography (ERG) would reveal rod and cone dysfunction
- Visual field defects and reflective dots may be visualized by spectral domain optical coherence tomography (sdOCT)
- Symptomatic vision impairment
BCD cannot be corrected by wearing glasses or contact lens and such vision impairment is known as ‘low vision.’ People with BCD have to be referred to low vision specialists or professionals who are trained in the management of visual impairment. Periodic monitoring is necessary to keep track of disease progression.
For those at higher risks it is possible to test for carriers during pregnancy, through prenatal testing.











